The Problem with Tylenol From a Functional Medicine Perspective
Sep 23, 2025Author: Jeffrey Wacks, MD
Acetaminophen (Tylenol) is widely considered to be a safe, first-line pain reliever and fever reducer. Recently, however, concerns have emerged regarding prenatal and/or infantile exposure to Tylenol and a potential link to autism. As the medical community and the general public begin having conversations about whether or not this is true, it will be helpful to integrate a functional medicine perspective so that we can understand the biological plausibility of this assertion as well as how to proceed with caution when we do need to take Tylenol if we are not sure whether it truly increases the risk of autism.
Bottom Line: Regular acetaminophen use can increase oxidative stress by depleting glutathione (the body's master antioxidant). This oxidative stress then creates a variety of issues including direct tissue damage and disruption of the gut microbiome. Your genetics likely increase your susceptibility to these problems, particularly if you have MTHFR mutations.
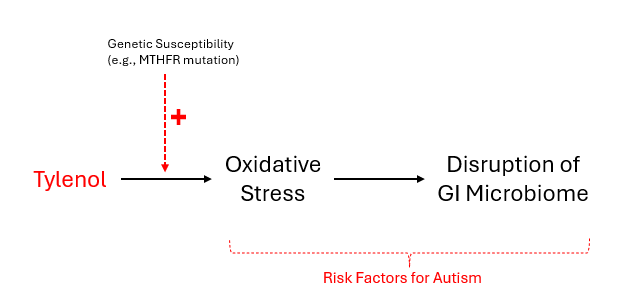
Figure 1. Model for the functional imbalances potentially caused by acetaminophen usage.
Could Tylenol Really Cause Autism? The Current Evidence
Is it really possible that Tylenol could cause autism? The honest answer is: we don't know for certain. A notable study done in 2020 by Ji et al.1 (aka Boston Birth Cohort) looked at biomarkers of fetal exposure to acetaminophen and showed a significantly increased risk of autism. This study is unique because it looks at biomarker data instead of exposure questionnaires, which would eliminate the problem of recall bias. Additionally, a 2025 meta-analysis by Prada et al.2 (aka Mount Sinai-Harvard Study) concluded that there was a positive association between acetaminophen exposure during pregnancy and increased incidence of autism. They identified 46 studies in their meta-analysis, of which 27 showed a positive association, 9 showed no association, and 4 showed a negative association. The authors noted that higher-quality studies were more likely to show a positive association. This recent meta-analysis was in line with previous meta-analyzed data,3,4 which showed an approximate 19% higher risk.
That being said, other studies show that it doesn't. For example, a 2024 population-based study by Ahlqvist et al.5 (Swedish Nationwide Cohort) showed that when controlled for familial compounding (analysis between siblings), the association was not observed. The counter-argument to this is that genetic susceptibility is part of the equation and should not be controlled for.
But if we are being scientifically honest, we have to at least admit that there is a possible association and at the very least it is unclear. It is fair to say that causality has not been definitively established, but we certainly cannot say definitively that an association has been completely ruled out. You cannot simply disregard meta-analyzed data without an explanation.
Additionally, the functional imbalances that are caused by Tylenol are also independently associated with autism:
- Oxidative Stress6,7 and Systemic Inflammation8,9
- Disruption of the GI Microbiome10,11,12
- MTHFR genetic polymorphism13,14,15,16,17
The Central Problem: How Tylenol Depletes Your Body's Master Antioxidant (Glutathione)
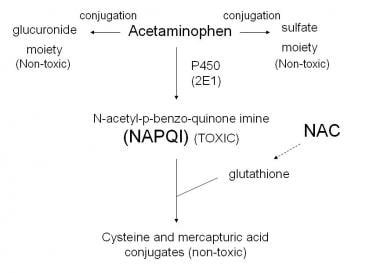
When you take acetaminophen, a small percentage of it is detoxified via cytochrome P450 oxidation to become NAPQI (N-acetyl-p-benzoquinone imine)—a highly reactive, toxic metabolite.18
Figure 2. Illustration of the detoxification pathway of acetaminophen. Emphasis on the NAPQI pathway being toxic and depleting glutathione levels. Courtesy of Wikimedia Commons.19
NAPQI itself is the source of toxicity, not acetaminophen. This reactive metabolite must be immediately neutralized by glutathione through a conjugation reaction. Each molecule of NAPQI consumes one molecule of glutathione during this detoxification process. This is where the problem begins. If either the dose of Tylenol is too high, the detoxification system is impaired for another reason, or there is a baseline depletion of glutathione, then significant oxidative stress will result (via direct toxicity and oxidative damage from NAPQI as well as depletion of the antioxidant defense system). The idea that Tylenol depletes glutathione levels is well-established, although this is generally only recognized as significant at supratherapeutic Tylenol dosages. However, some evidence suggests the decrease in glutathione may be significant even at therapeutic dosages.20
The key point: Tylenol itself is generally not the problem—it's the inevitable production of NAPQI and the consumption of glutathione during its detoxification that creates vulnerability to oxidative stress, especially when protective reserves are depleted.
Why Some People Are More Vulnerable: The MTHFR Connection
Understanding Your Body's Methylation System
The MTHFR gene produces an enzyme responsible for converting folate to its active form and regulating homocysteine levels through methylation—a fundamental biochemical process occurring millions of times per minute in every cell.
Why methylation matters for Tylenol safety:
- Helps detoxify acetaminophen through pathways separate from the toxic NAPQI pathway
- Maintains glutathione synthesis to keep your antioxidant defenses strong
Beyond Tylenol detoxification, methylation is essential for brain development:
- DNA methylation patterns that control gene expression during brain development
- Neurotransmitter synthesis (dopamine, serotonin, norepinephrine)
- Myelination of developing neural pathways
- Detoxification of environmental toxins during critical brain development
- Glutathione production for antioxidant protection
How Oxidative Stress Creates Inflammation and Tissue Damage
The degree to which this happens in routine clinical practice is debatable. However, at a biological level, it's well-established that oxidative stress (free radicals) triggers inflammatory cytokine production and can damage tissues—including brain tissue.21
During critical developmental periods, this process may:
- Damage developing neurons during crucial growth phases
- Disrupt the formation of neural connections
- Trigger brain inflammation that persists beyond the initial exposure
- Interfere with normal gene expression patterns needed for healthy brain development
It's also possible that manipulating prostaglandins (inflammation molecules) at critical moments affects brain development in ways we don't fully understand. There may be compounding effects when Tylenol is given repeatedly.22
The Gut-Brain Connection: How Tylenol Affects the Microbiome
Why Your Gut Microbiome Matters for Brain Development
Recent research reveals acetaminophen's significant impact on gut microbiome composition—a critical factor in neurodevelopment that functional medicine practitioners closely monitor. The gut-brain axis plays a fundamental role in brain development, immune system maturation, and behavioral regulation.
The microbiome's role in neurodevelopment:
- Neurotransmitter production (90% of serotonin is made in the gut)
- Immune system education and microglial programming
- Blood-brain barrier development and integrity
- Stress response system calibration
- Social behavior development through microbial metabolites
The Research Findings
Studies show that prenatal acetaminophen exposure was associated with reduced gut bacterial diversity in childhood and changes in key bacterial populations.23
Why this matters for autism risk: Children with autism consistently show altered gut microbiomes compared to typical children, including:
- Reduced bacterial diversity
- Increased harmful bacteria
- Decreased beneficial bacteria
- Altered metabolite production affecting brain function
- Increased intestinal permeability ("leaky gut")
Functional Medicine Solutions: Proceeding with Caution and Protection
The Precautionary Principle in Practice
Given the emerging research on acetaminophen and neurodevelopmental risks, functional medicine practitioners advocate for a precautionary approach—especially during pregnancy and early childhood. This doesn't mean never using acetaminophen, but rather:
- Using it judiciously and only when truly necessary
- Supporting the body's detoxification systems when use is required
- Addressing genetic susceptibilities before conception when possible
- Monitoring for early signs of systemic dysfunction
Protecting Against Acetaminophen's Systemic Effects
- Support Glutathione Production
- Consider administering N-acetylcysteine, oral liposomal glutathione, or topical glutathione in addition, if Tylenol is necessary.
- Other nutrients that support glutathione levels include: alpha-lipoic acid, selenium, other antioxidants, and milk thistle
- Optimize Methylation Status
- Methylfolate and/or methylcobalamin (Vitamin B12)
- Avoid folic acid supplements, cyanocobalamin
- Microbiome Support
- Consider probiotics, polyphenol-based prebiotics
- Additional Antioxidant Support
- Consider supplements such as curcumin, tocotrienol Vitamin E, whole food Vitamin C
Our Recommendations
Based on the emerging concerns about Tylenol and its potential link to oxidative stress and autism, we recommend:
- Use Tylenol in pregnant women and infants with caution
- If Tylenol is necessary, consider co-administering supplements that support glutathione levels (NAC, liposomal glutathione, or topical glutathione for children)
- Consider MTHFR genetic testing for all children. Those who test positive should take additional precautions, potentially including methylation support
- If concerned about Tylenol-induced oxidative stress, consider testing urine F2-Isoprostane/creatinine and/or stool markers of inflammation or microbiome dysfunction
The Bigger Picture: Making Informed Choices
Understanding acetaminophen's systemic effects doesn't mean never using it, but rather making informed decisions that consider both immediate benefits and long-term consequences. By supporting your body's detoxification pathways, addressing genetic susceptibilities, and maintaining a healthy microbiome, you can minimize potential harm while preserving treatment options when truly needed. But given the uncertainty around Tylenol's relationship to autism, if the general public and individual patients prefer to take a more conservative approach to Tylenol utilization during pregnancy and in infancy, then this seems reasonable. This will have to be weighed against the risk of the undertreated fever or underlying issue itself, but this will have to be done on a case-by-case basis.
The functional medicine approach emphasizes that no intervention occurs in isolation—every medication, supplement, and lifestyle choice affects multiple interconnected systems. By understanding these relationships, we can make choices that support both immediate symptom relief and long-term health optimization.
Key Takeaway: Acetaminophen use should be balanced with proactive support for glutathione production, methylation pathways, antioxidant defenses, and microbiome health—especially in individuals with genetic susceptibilities like MTHFR mutations..
References:
- Ji Y, Azuine RE, Zhang Y, et al. Association of Cord Plasma Biomarkers of In Utero Acetaminophen Exposure With Risk of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder in Childhood. JAMA Psychiatry. 2020;77(2):180-189. doi:10.1001/jamapsychiatry.2019.3259
- Prada D, Ritz B, Bauer AZ, Baccarelli AA. Evaluation of the evidence on acetaminophen use and neurodevelopmental disorders using the Navigation Guide methodology. Environ Health. 2025;24(1):56. Published 2025 Aug 14. doi:10.1186/s12940-025-01208-0
- Masarwa R, Levine H, Gorelik E, Reif S, Perlman A, Matok I. Prenatal Exposure to Acetaminophen and Risk for Attention Deficit Hyperactivity Disorder and Autistic Spectrum Disorder: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis of Cohort Studies. Am J Epidemiol. 2018 Aug 1;187(8):1817-1827. doi:10.1093/aje/kwy086
- Alemany S, Avella-García C, Liew Z, et al. Prenatal and postnatal exposure to acetaminophen in relation to autism spectrum and attention-deficit and hyperactivity symptoms in childhood: Meta-analysis in six European population-based cohorts. Eur J Epidemiol. 2021;36(10):993-1004. doi:10.1007/s10654-021-00754-4
- Ahlqvist VH, Sjöqvist H, Dalman C, et al. Acetaminophen Use During Pregnancy and Children's Risk of Autism, ADHD, and Intellectual Disability. JAMA. 2024;331(14):1205-1214. doi:10.1001/jama.2024.3172
- Chen L, Shi XJ, Liu H, et al. Oxidative stress marker aberrations in children with autism spectrum disorder: a systematic review and meta-analysis of 87 studies (N = 9109). Transl Psychiatry. 2021;11(1):15. Published 2021 Jan 5. doi:10.1038/s41398-020-01135-3
- Ismael HM, Ismail PA. Investigating Oxidative Stress and Impaired DNA Repair Capacity as Diagnostic Biomarkers in Autism Spectrum Disorder. J Mol Neurosci. 2025;75(3):93. Published 2025 Jul 28. doi:10.1007/s12031-025-02392-x
- Arteaga-Henríquez G, Lugo-Marín J, Gisbert L, et al. Activation of the Monocyte/Macrophage System and Abnormal Blood Levels of Lymphocyte Subpopulations in Individuals with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Int J Mol Sci. 2022;23(22):14329. Published 2022 Nov 18. doi:10.3390/ijms232214329
- Upthegrove R, Corsi-Zuelli F, Couch ACM, Barnes NM, Vernon AC. Current Position and Future Direction of Inflammation in Neuropsychiatric Disorders: A Review. JAMA Psychiatry. Published online July 9, 2025. doi:10.1001/jamapsychiatry.2025.1369
- Lewandowska-Pietruszka Z, Figlerowicz M, Mazur-Melewska K. Microbiota in Autism Spectrum Disorder: A Systematic Review. Int J Mol Sci. 2023;24(23):16660. Published 2023 Nov 23. doi:10.3390/ijms242316660
- Tao X, Li Z, Wang D, et al. Perturbations in gut microbiota in autism spectrum disorder: a systematic review. Front Neurosci. 2025;19:1448478. Published 2025 May 16. doi:10.3389/fnins.2025.1448478
- Wan Y, Zuo T, Xu Z, et al. Underdevelopment of the gut microbiota and bacteria species as non-invasive markers of prediction in children with autism spectrum disorder. Gut. 2022;71(5):910-918. doi:10.1136/gutjnl-2020-324015
- Fang Y, Cui Y, Yin Z, et al. Comprehensive systematic review and meta-analysis of the association between common genetic variants and autism spectrum disorder. Gene. 2023;887:147723. doi:10.1016/j.gene.2023.147723
- Li Y, Qiu S, Shi J, et al. Association between MTHFR C677T/A1298C and susceptibility to autism spectrum disorders: a meta-analysis. BMC Pediatr. 2020;20(1):449. Published 2020 Sep 24. doi:10.1186/s12887-020-02330-3
- Qiu S, Qiu Y, Li Y, Cong X. Genetics of autism spectrum disorder: an umbrella review of systematic reviews and meta-analyses. Transl Psychiatry. 2022;12(1):249. Published 2022 Jun 15. doi:10.1038/s41398-022-02009-6
- Rai V. Association of methylenetetrahydrofolate reductase (MTHFR) gene C677T polymorphism with autism: evidence of genetic susceptibility. Metab Brain Dis. 2016;31(4):727-735. doi:10.1007/s11011-016-9815-0
- Pu D, Shen Y, Wu J. Association between MTHFR gene polymorphisms and the risk of autism spectrum disorders: a meta-analysis. Autism Res. 2013;6(5):384-392. doi:10.1002/aur.1300
- Shingina A, Mukhtar N, Wakim-Fleming J, et al. Acute Liver Failure Guidelines. Am J Gastroenterol. 2023;118(7):1128-1153. doi:10.14309/ajg.0000000000002340
- Acetaminophen metabolism. Courtesy of Wikimedia Commons. https://commons.wikimedia.org/wiki/File:Acetaminophen_metabolism.jpg. Accessed September 23, 2025.
- Slattery JT, Wilson JM, Kalhorn TF, Nelson SD. Dose-dependent pharmacokinetics of acetaminophen: evidence of glutathione depletion in humans. Clin Pharmacol Ther. 1987;41(4):413-418. doi:10.1038/clpt.1987.50
- Vigo MB, Pérez MJ, De Fino F, et al. Acute acetaminophen intoxication induces direct neurotoxicity in rats manifested as astrogliosis and decreased dopaminergic markers in brain areas associated with locomotor regulation. Biochem Pharmacol. 2019;170:113662. doi:10.1016/j.bcp.2019.113662
- Lee YS, Kim H, Brahim JS, Rowan J, Lee G, Dionne RA. Acetaminophen selectively suppresses peripheral prostaglandin E2 release and increases COX-2 gene expression in a clinical model of acute inflammation. Pain. 2007;129(3):279-286. doi:10.1016/j.pain.2006.10.020
- Laue HE, Shen Y, Bloomquist TR, et al. In Utero Exposure to Caffeine and Acetaminophen, the Gut Microbiome, and Neurodevelopmental Outcomes: A Prospective Birth Cohort Study. Int J Environ Res Public Health. 2022;19(15):9357. Published 2022 Jul 30. doi:10.3390/ijerph19159357